Arenes, C6H5R
Links
Benzene
→ nitrobenzene
Reagents: conc nitric acid in the
presence of conc sulphuric acid.
Conditions: 50 - 55oC (not higher) with
constant agitation since the reactants are immiscible.
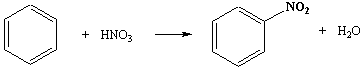
If the temperature rises above 60oC substantial further nitration occurs to 1,3-dinitrobenzene. Other arenes and substituted arenes require different conditions.
Alkanes
& alkenes
Arenes
Halogenoalkanes
Alcohols
Aldehydes and
ketones
Carboxylic acids
Acyl chlorides
Amines
Nitro compounds
Nitriles
Amides
Oxidation of alkylbenzenes
→ benzoic acid
Reagents: a solution of
potassium manganate(VII) and sodium hydroxide
Conditions: heat under reflux.
Homologues of benzene, that is benzene rings with alkyl side chains, give benzoic acid, irrespective of the length of the side-chain.
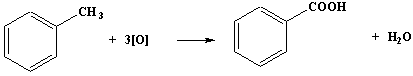
The initial product is sodium benzoate solution, which is made acidic with dilute hydrochloric acid to precipitate benzoic acid.
Benzene
→ alkyl benzene.
Reagents: chloroalkane in the presence of
anhydrous aluminium chloride.
Conditions: heat under reflux.
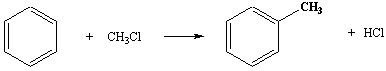
In practice further alkylation occurs to give 1,3-dimethylbenzene as well. Some reactions result in rearrangements of the alkyl groups, so extension of this reaction to other homologues is not as simple as it might appear. This is an example of a Friedel-Crafts reaction.
Benzene
→ aromatic ketone.
Reagents: acid (acyl) chloride in the
presence of anhydrous aluminium chloride.
Conditions: heat under reflux.
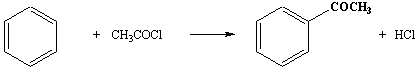
This is also a Freidel-Crafts reaction. Unlike the alkylation given above where the amount of aluminium chloride used is catalytic (i.e. small), this reaction needs a slight molar excess of aluminium chloride over the amount of ketone produced. This is because the ketone complexes in a 1:1 ratio with the aluminium chloride.