Amines RNH2 or ArNH2
Links
Amine
" amine salt.
Aliphatic and aromatic amines react similarly.
Reagent: dilute hydrochloric acid, or other mineral acid.
Conditions: at r.t.
CH3CH2NH2 + HCl " CH3CH2NH3+ + Cl -
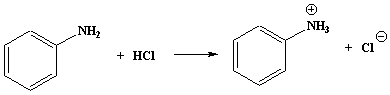
Amines that are insoluble in water will dissolve in hydrochloric acid, and be precipitated with alkali.
Alkanes
& alkenes
Arenes
Halogenoalkanes
Alcohols
Aldehydes and
ketones
Carboxylic acids
Acyl chlorides
Amines
Nitro compounds
Nitriles
Amides
Primary amine " diazonium
salt
Reagents: nitrous acid, produced from sodium nitrite and dilute
hydrochloric acid.
Conditions: Sodium nitrite is dissolved in water and
added slowly to a solution of the amine in excess concentrated hydrochloric acid
at 0o-10oC. Below 0oC the reaction is too
slow; above 10oC the diazonium compound will decompose.
Only diazonium salts from primary aromatic amines are synthetically useful, since aliphatic diazonium ions rapidly decompose:
C6H5NH3 + + HNO2 " C6H5N2+ + 2H2O
Coupling reaction of a diazonium salt with a phenol to give an
azo dye:
Reagents: a phenol such as phenol itself, or
2-naphthol, dissolved in sodium hydroxide solution.
Conditions: at r.t.
With phenol:
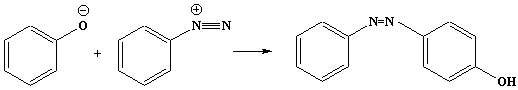
With 2-naphthol:
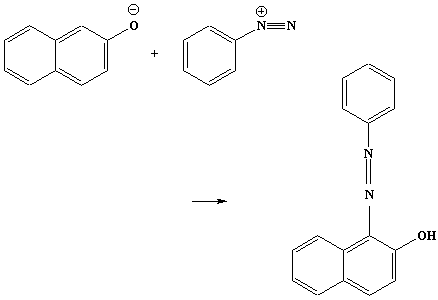
2-naphthol phenylazo-2-naphthol (red)