|
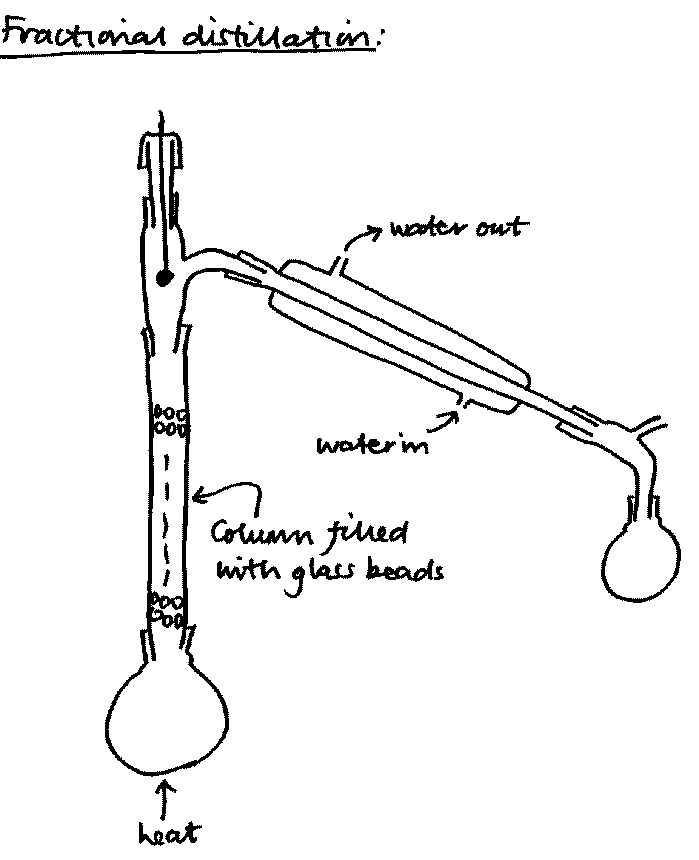
Fractional distillation is used to separate mixtures of volatile liquids. The round-bottomed distillation flask is underneath the fractionating column, which bears a stillhead and a thermometer adaptor. The stillhead leads to the water condenser (Liebig condenser) which is connected to the receiver by a vented delivery bend.
The fractionating column can be filled with almost anything that gives it a large surface area - glass beads or spirals, bits of broken glass tubing, ceramic rings. Alternatively it can be made with glass spikes sticking inwards (Vigreux column), or contain a spiral that forces the vapour around a helter-skelter pathway (Dufton column).
If a mixture of liquids forms an azeotrope, then that mixture cannot be completely separated by fractional distillation. This is covered further in the pages on Raoult's Law.
Chemistry Contents Simple distillation Reflux Gas preparation Drawing introduction