Answer 9 |
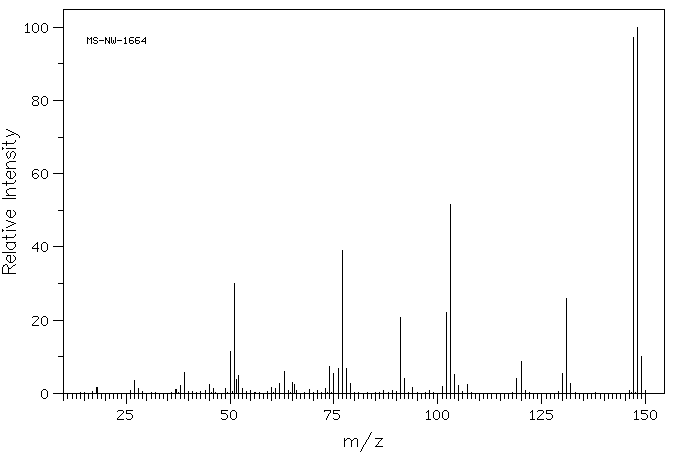
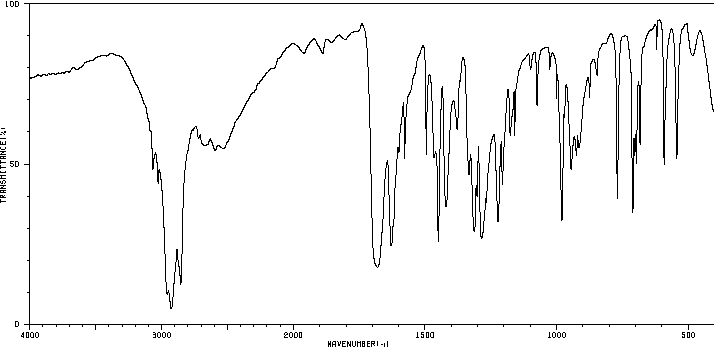
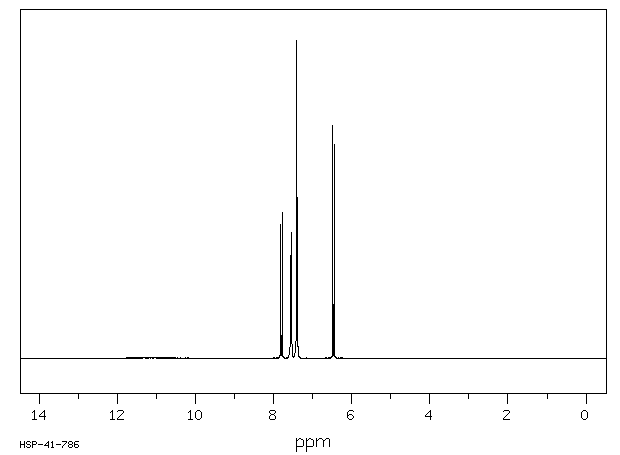
Since A effervesces when added to a solution of sodium hydrogen carbonate it is probably a carboxylic acid. It reacts immediately with bromine water in an addition reaction since the difference between C9H8O2 and C9H8O2Br2 is a bromine molecule. A therefore probably contains a C=C double bond. The relatively large carbon:hydrogen ratio suggests that the compounds contain a benzene ring – long chains of double bonds are unlikely. The existence of stereoisomerism means that the C=C bond has one group(s) other than hydrogen on each carbon atom. This leads to the conclusion that A is probably C6H5CH=CHCOOH, cinnamic acid, and B the dibromo derivative:
C6H5CH=CHCOOH + Br2 à C6H5CH(Br)CH(Br)COOH
The reaction is electrophilic addition.
With aqueous sodium hydroxide solution B is both neutralised and undergoes nucleophilic substitution:
C6H5CH(Br)CH(Br)COOH + 3NaOH à C6H5CH(OH)CH(OH)COONa + 2NaBr + H2O
C is therefore C6H5CH(OH)CH(OH)COOH, mandelic acid. This compound can be oxidised by acidified potassium dichromate solution to the diketone D:
C6H5CH(OH)CH(OH)COOH + 2[O] à C6H5COCOCOOH + 2H2O
D reacts with 2,4-dinitrophenylhydrazine but not with ammoniacal silver nitrate solution since ketones are not reducing agents. D gives steamy fumes with phosphorus pentachloride because of the –OH in the carboxyl group, the organic product being E, an acid chloride:
C6H5COCOCOOH + PCl5 à C6H5COCOCOOCl + POCl3 + HCl
With ammonia E gives F which is a nucleophilic substitution reaction:
C6H5COCOCOOCl + 2NH3 à C6H5COCOCOONH2 + NH4Cl
Both F and A are oxidised by hot aqueous potassium manganate(VII) solution to the sodium salt of G; this is benzoic acid, C6H5COOH. As with all carbon-containing side chains in aromatic compounds, potassium manganate(VII) oxidatively cleaves these to leave one carbon atom attached to the ring.
Mass spectrum:

Infrared spectrum:
Proton magnetic resonance spectrum:
Problem 9 Organic problems contents Home page