|
Pharmaceuticals | Epoxy resins | Fertilisers | Petrol and diesel | Esters, fats and oils
The new London specification for AS and A2 (first exams in January 2001 for AS)
The new specification (i.e.syllabus) contains sections on Applied Organic Chemistry; the changes from the previous syllabus are:
The present London syllabus (last exams in January 2002)
Section 23.2 of the Edexcel scheme deals with a number of applications of organic chemistry, some of which are not easy to find in textbooks. So here they are! You will find articles on this page concerning
Pharmaceutical compounds are increasingly designed to target particular receptors in tissues, based on known molecular structures. The compound is 'recognised' by the target tissue.
Molecular recognition employs structural features called pharmacophores, that are often similar to structures found in natural materials. An example is salbutamol, used as a bronchodilator to alleviate the symptoms of asthma. Noradrenaline is the natural bronchodilator, but it also increases heart rate and blood pressure. Salbutamol does not.
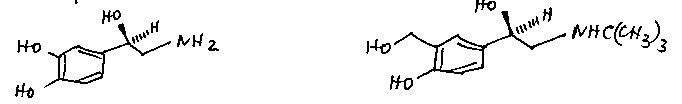
Noradrenaline Salbutamol
The pharmacophore in these molecules is the structure:
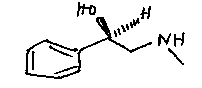
The content of the Edexcel syllabus is much simplerthen this; compounds forming H-bonds with water, because of polar groups for example, will be retained in watery tissue; so will salts, such as the anions of organic acids. Covalent compounds will favour fatty tissue.
Sorbitol, a sugar, will therefore be excreted readily since it has -OH groups which render it readily soluble in water; methyl mercury (CH3)2Hg is much more toxic than mercury(II) chloride since it is covalent and will be retained in fatty tissue or readily pass across fatty membranes.
No detailed knowledge of particular pharmaceutical compounds is expected.
This contrasts with inorganic fertilisers such as ammonium nitrate and ammonium sulphate. These have a rapid action but for osmotic reasons can cause burning and foliage decay in some plants.
Urea has the disadvantage, also shown by inorganic fertilisers, that there can be rapid leaching from soils due to its high solubility. This can cause problems in waterways.
'Natural' fertilisers such as 'hoof and horn' or dried blood do not share these defects, but are obviously impractical or undesirable for widespread use.
Epoxy resins.
Epoxy resins employ a smallish 'pre-polymer', itself a polymer, which is subsequently cross-linked into a hard, strong, glass-like and chemically resistant polymer by the addition of a hardener.
The most widely used epoxy resin is prepared from the following compounds by condensation polymerisation:
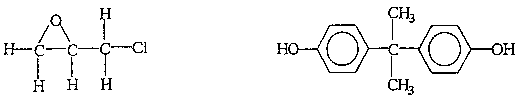
An excess of the epoxide monomer is used so that an epoxy group is left at each end of the pre-polymer chain.
The low molecular mass pre-polymer is of the form
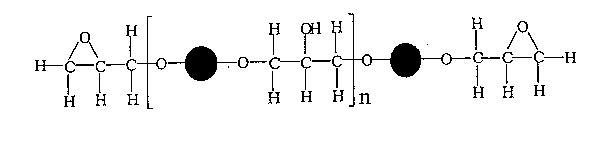
where: is shown as:

Before use the pre-polymer is mixed with the hardener, which is a diamine or a diamide, which then forms strong cross-links between the pre-polymer chains:
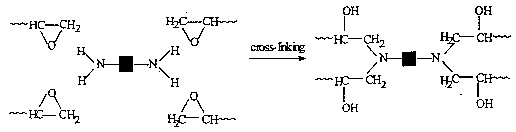
Such epoxy resins have no natural stickiness, so the bonded parts need support whilst hardening occurs. This can take 24 hrs or more.
Petrol and diesel.
Petrol (gasoline).
Two properties are of interest: the volatility and the octane number.
Volatility:
Octane number (RON):
Isomerisation:
This uses a Pt catalyst followed by separation and recycling of unchanged material. Thus pentane (RON 62) can be converted to the branched-chain isomer 2-methylbutane with RON 93.
Reforming:
This uses a Pt/Re catalyst (maybe £5 million worth in one reformer) which can convert alkanes to cycloalkanes, and cycloalkanes to aromatics. Thus hexane (RON 25) can be converted to cyclo-hexane (RON 83), cyclo-hexane to benzene (RON 106), and methylcyclohexane (RON 70) to methylbenzene (toluene; RON 120).
Cracking:
Heavy oils (C30 – C40) are heated over a catalyst in a fluidised bed, which gives
The conditions and the nature of the catalyst are varied to give the desired products.
Auto-ignition or pre-ignition.
Solving pre-ignition:
[Thanks to Christopher Cassano for extra information on this topic.]
Diesel.
Diesels use a heavier fuel, C15 – C 19, that is intended to auto-ignite