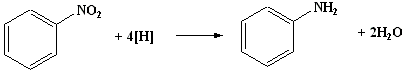- Place 2.1cm3 of nitrobenzene and 5 g of granulated tin in
a 150cm3 round-bottomed flask; fit the flask with a reflux
condenser.
|
|
- Pour 10 cm3 of concentrated hydrochloric acid down the
reflux condenser; heat the mixture over a gauze for 15 minutes.
|
- What is the reaction between the tin and hydrochloric acid?
- Sn + 2HCl → Sn2+ + 2Cl- + H2
- What is the reducing agent, and to what is it oxidised?
- The reducing agent is Sn2+, which is oxidised to Sn4+.
|
- Cool the flask and add a solution of 7.5 g of sodium hydroxide in 10
cm3 of water to redissolve the initial precipitate. Add about
15cm3 of water and rearrange the apparatus for distillation.
|
- What is the initial precipitate, and what does it become on
addition of excess sodium hydroxide?
- Tin(IV) hydroxide Sn(OH)4. This is amphoteric, and with
more alkali forms the soluble Sn(OH)62- ion.
- Why is more water added to the solution?
- This enables a technique called steam distillation. In the
large-scale preparation steam is blown through the mixture; in this one
the steam is generated in situ. Phenylamine distils over with the
steam; any unchanged nitrobenzene and the inorganic materials do not.
|
- Heat the mixture over a gauze and collect the cloudy distillate in a
small flask, stopping when the condensate becomes clear (about 18 cm3
will have been collected).
|
- Why is the initial distillate cloudy?
- It is a mixture of water and phenylamine which formas a cloudy
emulsion
- What is the clear condensate?
- Water; by now all the phenylamine has distilled over.
|
- Add 3 g of powdered sodium chloride to the distillate, shake to
dissolve, and then transfer the liquid to a separating funnel. Add about
4 cm3 of ethoxyethane and shake, relieving the pressure
occasionally. Allow the layers to separate, then run off the lower
aqueous layer into a small beaker. Transfer the ethoxyethane layer to a
small conical flask. Repeat the extraction of the aqueous layer with a
further 4 cm3 of ethoxyethane, and combine the ethoxyethane
extracts.
|
- What is the purpose of adding sodium chloride?
- Phenylamine is significantly soluble in water, but very much less so
in saturated sodium chloride solution. This process is called 'salting
out'.
- What is the function of the ?
- Solvent extraction; phenylamine is much more soluble in ethoxyethane
than it is in water.
- Why are two portions of 4 cm3 used, rather than a
single 8cm3 portion of of ethoxyethane?
- The theory of solvent extraction is a branch of equilibrium. It can
readily be shown that any solvent extraction is more effective if a
given volume of extracting solvent is used in several portions rather
than in a single one.
|
- Dry the ethoxyethane extract with two or three pellets of potassium
hydroxide. Decant the dried solution into a small pear-shaped flask, add
two or three anti-bumping granules, and assemble the flask into a
distillation apparatus incorporating a 0-220oC thermometer.
Distil off all the ethoxyethane using a beaker of hot water for heating
having ensured that all flames in the laboratory have been extinguished.
Remove the ethoxyethane from the vicinity of the experiment.
|
- Suggest a reason for using KOH as a drying agent, rather than the
more conventional calcium chloride or sodium sulphate.
- The use of potassium hydroxide would also eliminate any traces of
hydrochloric acid in the phenylamine.
- Why must all flames in the laboratory be extinguished?
- The vapour of ethoxyethane is very dense and will creep along
bench-tops over a considerable distance. It is possible for an explosion
to ensue even if the source of ignition is several metres away from
where the ethoxyethane is being used.
|
- Run the water out of the condenser, and distil the phenylamine
heating with a small flame. Try to keep phenylamine condensing on the
thermometer for about a minute before allowing any to distil over.
Collect the fraction boiling between 180o and 185oC.
|
- Why is the water run out of the condenser?
- Phenylamine has a sufficiently high boiling temperature for an air
condenser to be efficient at condensing the vapour
- Why is the phenylamine kept condensing on the thermometer bulb
before allowing distillation to proceed?
- This allows the thermometer to come into thermal equilibrium with
the vapour - a general necessity in distillation, but needing more care
than usual if the boiling temperature of the distillate is high.
Phenylamine boils at 184oC.
|