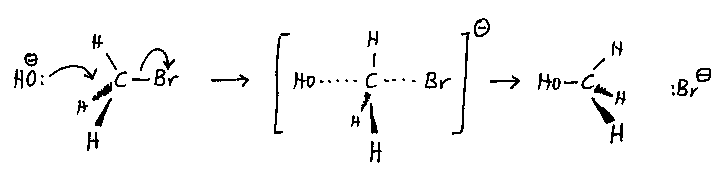Typical reaction: the reaction of hydroxide ions with bromomethane in
aqueous ethanol solvent. The mixture is heated under reflux.
The mechanism:
Points:
- The approach of the nucleophile is along the C-Br axis, which is the approach of lowest
energy.
- The use of a chiral halogenoalkane gives inversion (the Walden inversion) of the
molecule, so the product alcohol is optically active.
- There is no intermediate; the negative ion is a transition state, and the reaction is a
one-step reaction.
- The mechanism is favoured by halogenoalkanes which would give primary carbocations if SN1
were the pathway, and by a decreased solvent polarity.
Reaction mechanisms index
Home Page