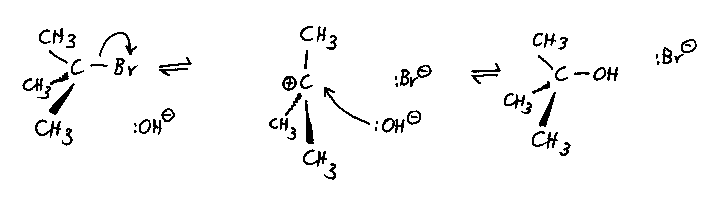
Typical reaction: the reaction of 2-bromo-2-methylpropane with
hydroxide ions, using a solvent of aqueous ethanol. The mixture is heated under reflux.
The mechanism:
Points:
- This mechanism has a true intermediate, the carbocation; the formation of this is the
rate limiting step.
- The carbocation is planar. In principle this suggests that attack from the nucleophile
is equally likely from either side, so that if the starting halogenoalkane was chiral, the
product alcohol would be a racemic mixture of the two mirror images and would not show
optical activity.
- In practice there is some steric hindrance from the departing bromide ion, and the
product mixture is roughly 60% inverted configuration, 40% retained.
- This mechanism is favoured by a polar solvent, and by branching of the carbon chain so
that the carbocation formed is a tertiary carbocation.
Reaction mechanisms index
Home Page