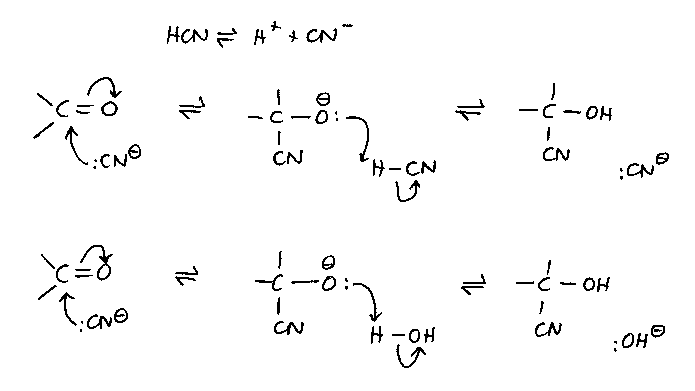Typical reaction: addition of hydrogen cyanide to an aldehyde or some
ketones. Potasium cyanide in aqueous ethanol at pH8 is heated under reflux with the
carbonyl compound.
The mechanism:
Points:
- The pH is 8 because if it is lower, there is too little cyanide ion present for the
first step; if it is higher, there is too little tendency for the anionic species to pick
up a proton and form the -OH group.
- The mechanism shows the proton coming wither from HCN or from water.
- There are some nucleophilic additions to C=O that involve attack of a proton on the
carbonyl oxygen to give a carbocation that is then attacked by the nucleophile.
- Few ketones give the reaction - see the Organic Reactions
Catalogue pages.
Reaction mechanisms index
Home Page