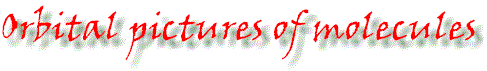
|
Methane and its isoelectronic molecules:
|
Methane: the carbon atom has four pairs of electrons, all bonding. The orbitals are tetrahedrally-disposed, and this is also the shape of the molecule with all angles equal at 109o 28' (about 109.5o). |
|
Ammonia: nitrogen also has four pairs of electrons, but three are bonding and one is non-bonding or a lone or unshared pair. The molecule's shape is trigonal (or triangular) pyramidal, the shape being defined by the atom centres. It is still fundamentally a tetrahedral disposition of electron pairs because there are four of them. The lone pair is fatter than the bonded pairs, and this reduces the H-N-H bond angle from the tetrahedral angle of 109o 28' to about 107o. |
|
Water: also based on tetrahedrally-arranged electron pairs, the water molecule is bent. Two lone pairs compress the H-O-H angle from the tetrahedral value to about 104o. |
Ethane and its isoelectronic molecules:
 |
Ethane: each of the carbon atoms has tetrahedrally-disposed orbitals. |
 |
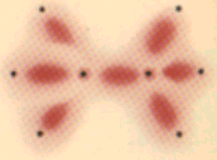
Hydrazine N2H4: this has the same electronic arrangement as ethane, but each nitrogen has one lone pair. |
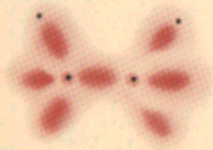 |
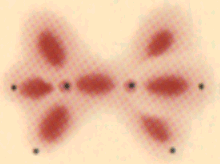
Hydrogen peroxide H2O2: in this molecule each of the oxygen atoms has two lone pairs. |
Ethene:
|
Ethene: only the pi-bond is shown in the C=C bond. It shows why the molecule is receptive to electrophiles! |
Orbitals contents Orbitals and reactions Chemistry contents Home Page