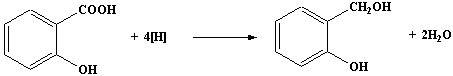- Assemble in a fume cupboard a 3-necked flask fitted with a stirrer,
a reflux condenser and a tap funnel, the apparatus being thoroughly dry,
and the condenser and the funnel closed by calcium chloride guard tubes.
|
- Why must the apparatus be thoroughly dry?
- Much of the preparation technique is concerned with preventing the
dangerous reaction of lithium aluminium hydride with water - the
reaction is exothermic and generates hydrogen, so the danger of
explosion is evident.
- What is the function of calcium chloride guard tubes?
- Anhydrous calcium chloride has a high affinity for water, and the
guard tubes prevent water vapour from entering from the atmosphere.
Lithium aluminium hydride dust can even catch fire on a damp day.
|
- Run 90 cm3 of dry ethoxyethane into the flask and start
the stirring. Weigh out 2.5g of lithium aluminium hydride, and then
divide 0.5 g of this amount into very small portions: add these
portions in turn cautiously to the stirred ethoxyethane. Then add the remaining 2.0g of the hydride more rapidly.
|
- How is ethoxyethane dried?
- Sodium wire is used.
- What is the reason for adding a small portion of lithium
aluminium hydride initially?
- This is to remove any traces of water which in spite of the
precautions taken may be present in the reaction flask.
|
- When the addition is complete, continue stirring the mixture for 15
minutes.
|
- What is the reason for this stirring?
- To ensure that the lithium aluminium hydride is dissolved.
|
- Now cool the mixture thoroughly in an ice-water bath, and run in
over a period of 45 minutes a solution of 6.0g of dry
2-hydroxybenzoic acid in dry ethoxyethane.
|
- Why is the mixture cooled?
- The reduction is exothermic; the boiling temperature of ethoxyethane
is 34.5oC and the reaction must at this stage be kept cold to
prevent thermal runaway and loss of the ethoxyethane.
|
- When the addition of the solution of 2-hydroxybenzoic acid is
complete, heat the mixture under reflux on the water bath for 15
minutes.
|
- Why is the mixture heated for 15 minutes?
- This is to ensure completion of the reduction and the consumption of
the lithium aluminium hydride.
|
- Thoroughly chill the mixture in ice-water, and hydrolyse any unused
hydride by the slow addition of 50 cm3 of ordinary
undried ethoxyethane, followed by the slow addition of 75 cm3
of dilute sulphuric acid.
|
- Why does the mixture have to be chilled?
- The reaction of lithium aluminium hydride with water is very
exothermic.
- Why is the addition of the undried ethoxyethane slow?
- To ensure that the reaction with water is not violent.
- Why is the addition of sulphuric acid slow?
- The is precautionary, in case any lithium aluminium hydride still
remains.
- What is the purpose of the sulphuric acid?
- The hydrolysis of lithium aluminium hydride also gives lithium
hydroxide, which is not very soluble in ethoxyethane and gives a white
sludge. Sulphuric acid forms water-soluble lithium sulphate which passes
into the aqueous layer.
|
- Transfer the reaction mixture to a separating funnel, run off and
keep the
lower aqueous layer and retain the ethoxyethane layer in the funnel. Shake the aqueous
layer successively with two 25cm3 portions of ethoxyethane, and add
these to the main ethoxyethane solution. Dry the ethoxyethane solution with
anhydrous sodium sulphate.
|
- Why is the aqueous layer shaken with ethoxyethane?
- This extracts any of the organic product that remins in the aqueous
layer.
- Why is the organic product significantly soluble in the aqueous
layer?
- The two hydroxyl groups make it significantly soluble since they can
hydrogen bond with water.
- Why are two 25 cm3 portions of ethoxyethane used,
rather than one of 50 cm3?
- The process of solvent extraction is more efficient if several
extractions are made rather than just one.
- How would you know when the solution is dry?
- It would be clear rather than cloudy.
|
- Filter the ethoxyethane solution, which is then distilled to remove
ethoxyethane. Arrange a distillation apparatus using a 100 cm3
distilling flask heated with a hot water bath (NO naked flames should be
anywhere in the laboratory) and fitted with a tap funnel. The
ethoxyethane
solution is placed in the funnel, and added to the heated distillation
flask as fast as the ethoxyethane distils over. When all the
ethoxyethane has
distilled over, cool the oily residue of crude product, which will
rapidly crystallise.
|
- Why is the solution filtered?
- This removes any particles of sodium sulphate.
- Why should there not be flames anywhere in the laboratory?
- The vapour of ethoxyethane is very dense, and can creep along bench
surfaces or along the floor. It is possible to ignite it if there are
flames on the other side of the room.
|
- Recrystallise the product twice from the minimum amount of boiling
pure water; cool the saturated solution to about 70oC, then
in ice-water. Filter the final product and dry in a desiccator. The
product gives fine white crystalline plates, m.p. 84-85oC.
|
- Why is the minimum amount of boiling water used?
- The solution needs to be saturated at the boiling temperature of the
solvent to minimise losses.
- Why is a desiccator used rather than drying the crystals in an
oven?
- The melting temperature of the product is low and it would melt; a
desiccator is in any case much more efficient.
|