 |
|
| Water is very common (see the table below), essential for living things, a substance that has been widely studied. It still attracts some controversy concerning its structure and properties. I want to talk about ice, since its structure seems to contradict common sense.
The properties of water generally are dominated by the hydrogen bond, the electrostatic bond between the hydrogen that is d+ because of electron withdrawal by the more electronegative oxygen, and the d- on the oxygen atom of another molecule:
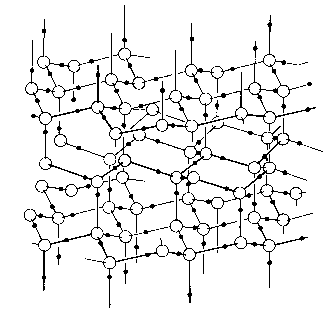
This bond has a strength of around 20 kJ mol-1 in water, that is about one-twentieth of the sort of covalent bonds found in organic compounds or the O – H bond in water itself, 464 kJ mol-1. The hydrogen bond linkage always has the three atoms in a line, or very nearly so. So what is the problem with ice? There are several forms of it, some of which are solid above 80oC, but I am referring to the ‘ordinary’ ice that you get out of the ‘fridge. This ice is less dense than the liquid; a solid being less dense than its parent liquid is a property that is very rare. Most solids are denser than the liquids from which they crystallise. The lower density of ice compared with water is the result of an increase in hydrogen bonding as the solid forms from the liquid. The molecules align and form into rings of six, with the oxygen atoms at the vertices of the hexagon (see the head of the page for the structure). The crystal is built up from layers of these rings, in a structure reminiscent of diamond and in fact closely related to the silicate structure tridymite. Each oxygen atom is surrounded by four others in a regular tetrahedral arrangement. The O-H-O group is not quite linear, the hydrogen being off linearity by a couple of degrees. The hydrogen bonds space the molecules in the solid further apart than they are in the liquid. As ice melts, some of the hydrogen bonds are thermally disrupted, and the molecules move closer. This is pretty standard stuff. Have you ever wondered why, since the hydrogen bond is an attractive force, it pushes molecules further apart in ice? The problems of explaining structure, indeed many properties in chemistry, come from the fact that any given trend in properties is affected by several factors. In the case of intermolecular forces, we talk mainly about the dominant type and forget that there are several forces operating. Crucially, we often forget to talk about the repulsion that there is between molecules. In the case of ice, the balance of attractive forces and repulsive forces gives rise to the expanded lattice. The hydrogen bonds do attract, but their maximum attraction requires (near) linearity, and in addition there are the repulsive forces between electron pairs to consider. When all of the forces are taken into account, not just the H-bonds, then the lowest energy structure for water has the open structure that gives it its surprisingly low density. This has a strong sense of post hoc about it; it sounds as though we are fiddling the forces to explain the observed structure. A lot of chemical explanation sounds like that, and the reason lies in the multiple nature of the factors that affect any given property. Furthermore, small changes in one or other of these factors can lead to huge changes in observed properties, so that the prediction of the bonding or structure of some new compound is extremely difficult.
|
||||||||||||||||||||||||||||||||||||||||||||