The typical reaction is that of an alkene with bromine, either in the
gas phase or with bromine dissolved in some inert organic solvent such as
tetrachloromethane; both at room temperature.
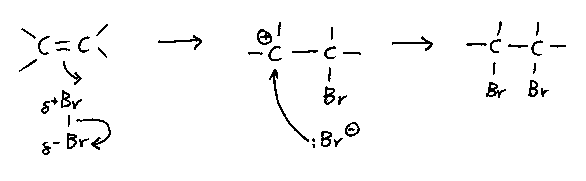
The mechanism:
Points:
- Bromine is not polar, but becomes polarised, and hence electrophilic, by the electron
density in the C=C bond.
- Draw the + charge of the carbocation out of the way of the curly arrow showing the
incoming electron pair from the nucleophilic bromide ion.
- The mechanism involves a bridged 'bromonium' ion in reality, but this is not addressed
at A level.
Reaction mechanisms index
Home Page