Ammonium ion can be determined by treatment of a solution of the test material with excess sodium hydroxide solution; the ammonia produced is then distilled into a known excess of standard hydrochloric acid. The residual acid is titrated against standard alkali.
This method is more time-consuming than some, but does have a number of advantages from the point of view of teaching practical techniques.
Method.
1 Weigh accurately about 1.5 g of ammonium chloride, and transfer with the aid of a little water to a 100 cm3 round-bottomed Quickfit flask.
2 Add 50 cm3 bench sodium hydroxide solution to the flask.
3 Into a crystallising dish, beaker or evaporating basin of suitable size pipette 50.0 cm3 of 1.00 mol dm-3 hydrochloric acid.
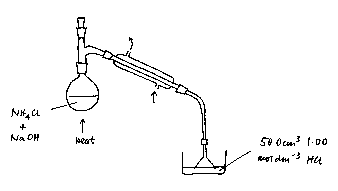
4 Set up the following apparatus. The funnel should just dip under the surface of the acid in the receiver; this is to prevent suck-back.

5 Distil the mixture gently until about two-thirds of the original solution has distilled over. Then rinse off the funnel and transfer the solution from the receiver quantitatively to a 250 cm3 graduated flask. Make to the mark with pure water and mix well.
6 Titrate 25.0 cm3 portions of this solution with 0.100 mol dm-3 sodium hydroxide solution using phenolphthalein indicator. Repeat to obtain three consistent titres.
Results.
Mass of ammonium chloride taken/g:
| Final volume/cm3: | ||||
| Initial volume/cm3: | ||||
| Titre/cm3: |
Mean titre/cm3:
Hence calculate the percentage purity of the ammonium chloride used.