A chemical attractant from amanita muscaria
J R G Beavon
Introduction
The role of pheromonal attraction (or repulsion) is well-documented in insect species, but
apart from the obvious role of flower scents as attractants for insects the role of other
chemical attractants is poorly documented. An early paper by Sforz (1907) failed, due to
poor analytical techniques, to show the presence of an attractant in Amanita phalloides;
since then the field has been almost wholly neglected.
In this study of the attractants from Amanita spp, concentrated solutions were used in field experiments, the unusual nature of the tests necessitated by the failure to breed the attracted organism in captivity. Because of the relatively short fruiting season for Amanita, some fungi grown in the laboratory were used. The fungus is essential; impregnated filter-paper strips were used as well, and although the organisms were attracted they appeared confused by the absence of the caps and the usual physiological effects were not seen.
Experimental methods and results
Preliminary extraction involved the homogenisation of 1kg of fungal cap at 0░C in 50cm3
of acetate buffer at pH5, followed by centrifugation to deposit the cell fragments.
The solid pellet was then freeze-dried. The dry, greyish-brown powder was then extracted
with ice-cold ether in two portions, each 20cm3 per 5g of dried residue. The
extract was centrifuged, and the pale-yellow ethereal solution evaporated under reduced
pressure in a rotary evaporator. The residue was taken up in 1cm3 of puriss
ether.
Chromatographic analysis using 0.2mm silica gel thin layers and a chloroform solvent showed two fluorescent spots under UV light at 354nm, one intensely green at RF 0.39 which turned out to be the active principle, and another, pale blue and faint, at RF 0.95. The chromatography was scaled up to preparative levels, and 0.12g of pale yellow powder was obtained.
Testing in the field
Caps of A. muscaria were grown according to the method of Mycotta et al (1981);
each cap was inoculated with an ethereal solution of the compound, containing 1, 5 or
10Ámol, and the caps then taken to the experimental area. This is a small copse, mainly
of oak and a little ash, and well-removed from public influence at OS ref OV302569. The
caps were laid out according to the randomisation procedure of Chimolowski (1986), in the
late afternoon. The organisms concerned are mainly nocturnal. Data on the number of
organisms attracted, and those showing pronounced response to the attractant were analysed
according to the algorithm of Grk (1986). These results are given in Table 1. The
different morphologies are designated Type A and Type B in this table.
| Date | Sunset time | Time of appearance | Y, Ámol | Max. number of Type A | Max. number of Type B | Number starting activity | Number finishing activity | Number not involved |
| 7 March | 17:51 | 17:30 | 1 | 13 | 17 | 12 | 12 | 18 |
| 8 March | 17:53 | 17:45 | 5 | 6 | 4 | 8 | 6 | 2 |
| 9 March | 17:56 | 17:39 | 10 | 16 | 17 | 32 | 32 | 1 |
| 21 March | 18:14 | 17:30 | 10 | 20 | 25 | 40 | 40 | 5 |
| 22 March | 18:16 | 18:30 | 10 | 1 | 2 | 2 | 2 | 0 |
| 11 April | 19:50 | 19:53 | 10 | 25 | 22 | 40 | 38 | 7 |
| 14 April | 19:57 | 22:30 | 10 | 60 | 60 | 120 | 120 | 0 |
Table 1: experimental results from field trials
Identification of the pale-yellow compound Y
Elemental analysis gave C 88.9% and H 11.1%; mass spectroscopy showed a molecular ion peak
at m/e 378. This data indicates a molecular formula of C28H42.
I.R. spectra showed bands at 960-970cm -1 and 1295-1310cm -1,
suggesting trans substituted alkene groups.
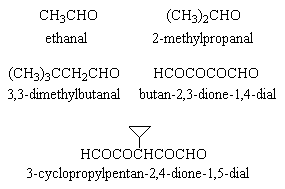
Reduction with hydrogen and platinum at room temperature showed an uptake of 6 moles of hydrogen per mole of Y. Ozonolysis resulted in five compounds (Fig 1); on the basis of this, and a successful synthesis of Y (Beavon, unpublished work), structure I (Fig 2) is that of Y, viz
1-cyclopropyl-2-propenyl-3,4-di(3-methylbut-1-enyl) cyclopentadiene
The trivial name pyxiflavin is recommended.

Figure 1: compounds obtained by ozonolysis of pyxiflavin
|
|
|
| I, pyxiflavin | II | III |
Figure 2: pyxiflavin and derivatives
Discussion
It is clear that pyxiflavin has considerable attractive and physiological effects for Gnomo
sapiens when associated with A. muscaria. The males and females of this species
differ morphologically so that identification at a distance is generally reliable. Usually
morphologically distinct pairs of G. sapiens on exposure to pyxiflavin spend some
time running around, then shed their plumage and fall over. The physiological picture then
becomes confused, but seldom lasts longer than 20 minutes. Further investigation of the
exact nature of the activity is in progress. The population of individuals in initial
experiments was small, but increased rapidly as time went on, and some form of
communication cannot be ruled out.
A derivative of pyxiflavin, II, synthesised in this laboratory, appears to be without effect; on the other hand III, which has so far received limited field tests, seems much more effective than pyxiflavin itself. This group of compounds, apparently effective for Gnomo spp., has provisionally been named the Pherognome group. It is believed that these substances are biosynthesised from acetate via mevalonic acid, and this idea is being pursued in this laboratory at present (Beavon and Johnson, in progress). It will also be of interest to see whether Pyxiflavin is active for other nocturnal wood-dwelling species, especially Pyxidae and Elvidae spp.; however these species are considerably more elusive and difficult to study than Gnomo, some workers even doubting their existence.
Bibliography
Chimolowski, A.V.: J. Stat. Res. 10, 16-118 (1976).
Grk, J. Comp. Sci. Sverdlovsk, 22, 131-8 (1986).
Mycotta, I.C.I., Russula, E. and Paxillus, A.: Helvetica Mycologica Acta, 2,
344-359, (1981).
Sforz, W.: Zeitschrift fur Animalische Forschung, 29, 82-8,
(1905).
Reprinted from Hooke issue 7 (January 1996), the science magazine of Westminster School; most of the articles are rather more serious than this one. It is available on-line.
Westminster School Chemistry contents Home Page