 |
- The nitronium ion NO2+ approaches the benzene ring
- A partial bond forms between the ring and the NO2+, the electron
density coming from the ring system
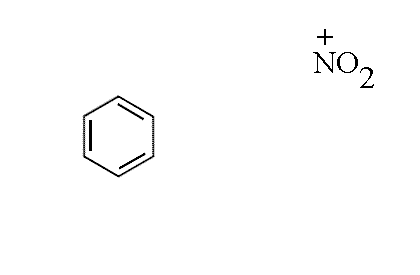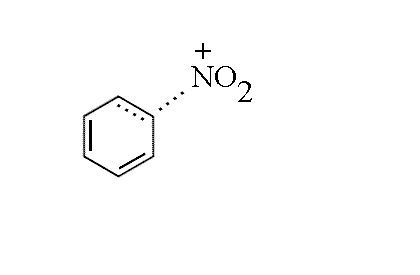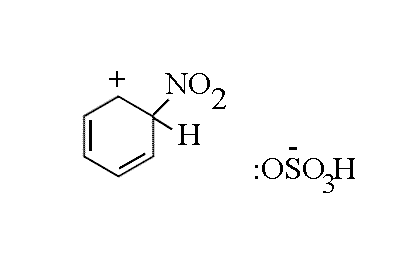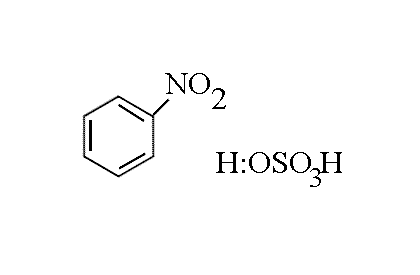
- An intermediate (the Wheland intermediate) forms, where the attacked carbon is sp3
hybridised and the delocalised electron system in the ring is broken
- The hydrogen ion that is to be lost must be accepted by the HSO4-
ion which moves towards the intemediate
- HSO4- accepts the proton, reforming sulphuric acid
- The electrons that bound the hydrogen return to the p-system in the ring, reforming the
delocalised system and restoring aromaticity.
|