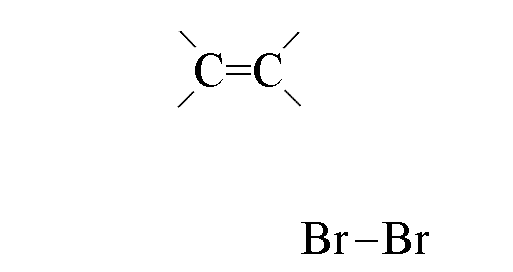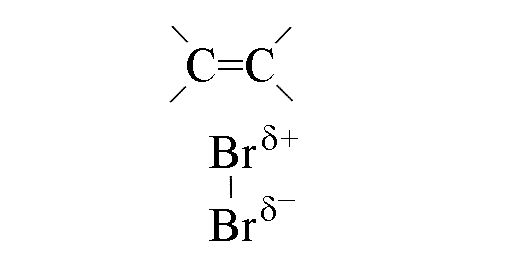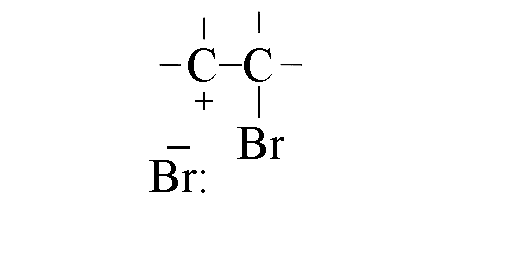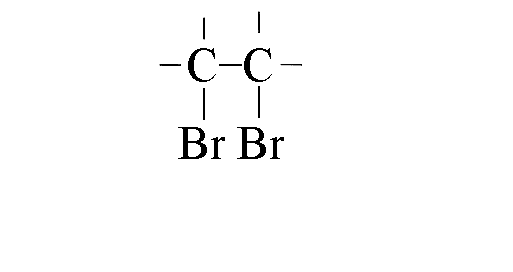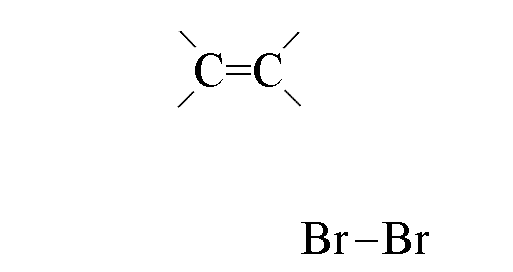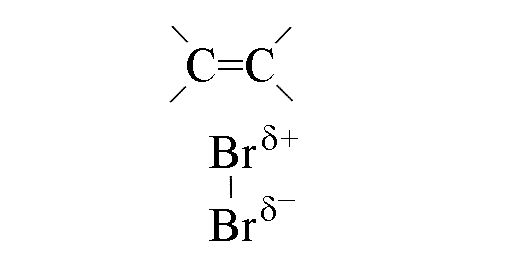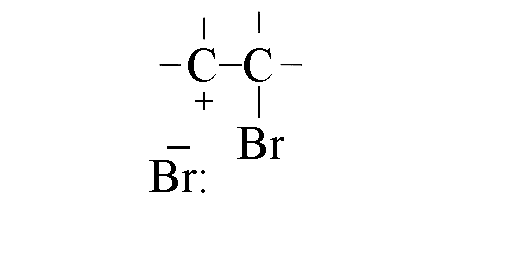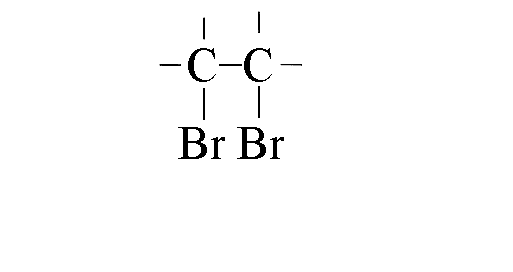 |
- Bromine molecules approach the electron-rich double bond
- The electrons in the double bond repel those in the bromine-bromine bond, polarising it
and making the bromine electrophilic
- As the C-Br bond forms the p-bond in the double bond weakens,
as does the s-bond between the bromine atoms
- The carbocation formed is attacked rapidly by the bromide ion to form the final product
|